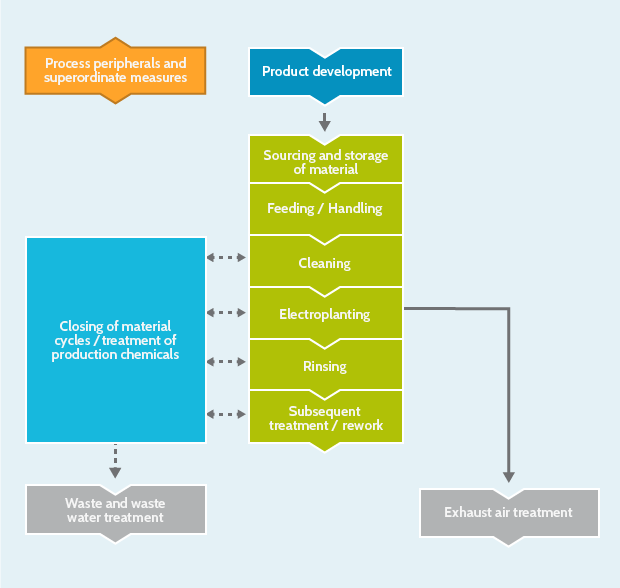
Product development
Best Available Techniques
Choice of manufacturing process prior to surface treatmen
- a different pressing oil, which may become pressed into the substrate micro-sturcture, might not respond to normal degreasing processes
- reduction of oil and grease applied in the mechanical production area via: use of volatile lubricants, employment of minimal quantity cool lubrication, dripping off and/or centrifuging the workpieces, pre-cleaning the workpieces at the point of production, shortening the storage time, drilling with compressed air cooling, use of applied plastic film lubricants in pressing.
- reduction of reworking
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 200, p. 215
right balance of specifications for workpieces
combination of thicker and thinner coating thickness required, as the deposits build preferentially at edges and corners of the workpiece and/or substrate (where the charge density is greatest) --> avoids flaking if the edges are subsequetly manupulated
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 199
Closing of material cycles / treatment of production chemicals
Best Available Techniques
Activated carbon treatment
Activated carbon filtration is an absorption technique (or adsorption, in the case of activated carbon) and used with filtration. Organic decomposition products in electrolytic solutions tend to disturb electrolytic metal deposition or the properties of the metal deposit itself. The major proportion of such products can be extracted from electrolytes through activated carbon treatment. The quantity of active carbon needed depends on the quantity of the products to be removed: up to 10 g/l may be necessary. Activated carbon is mixed into the electrolyte and removed by filtration after a suitable reaction time. A combination of normal filtration and active carbon cartridges in by-pass is used to continuously remove both solid contaminants and soluble organic decomposition products from the electrolytes. The process extenses of process solution life. Theoretically, contaminated activated carbon can be regenerated, but this is usually not economical or has associated limiting cross-media effects. The process is widely used, and frequently for bright nickel electrolytes.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 314
Anodising caustic etch recovery
A hot solution of sodium hydroxide creates a decorative matt surface finish by removing a thin layer of aluminium. This etching process is caused by a reaction between the aluminium and caustic soda that produces sodium aluminate and hydrogen gas. The etching process is typically responsible for 80 - 90 % of the aluminium in the waste treatment system. Chemical stabilisers (complexing agents) are added to prevent the aluminium from precipitating out in the etch tank. Water is used to rinse the etching solution off the parts. The rinse-water carries dissolved aluminium and caustic to the plant waste treatment system. If stabilisers are not used, the sodium aluminate concentration becomes too high and it will hydrolyse to produce alumina trihydrate, liberating free caustic soda. Diese Reaktion wird zur Aluminiumerzeugung genutzt. This reaction, known as the Bayer process, is used in the primary aluminium industry to make alumina. If not properly controlled, it leads to an accumulation of a rock-hard aluminium hydroxide scale in the etch tank. A regeneration system recirculates the etch solution continuously between the etch tank and a separate crystalliser tank, where the etch solution is seeded with alumina crystals in a separate crystalliser tank. It is then possible to regenerate the etch solution without scale building up. The hydrated alumina crystals formed in the crystalliser settle out in a settlement section. Regenerated etch solution, with reduced aluminium and increased free caustic levels, feeds back to the etch bath directly from the top of the crystalliser. Alumina crystals are withdrawn periodically from the bottom of the crystalliser and dewatered in a vacuum filter.
Regeneration can reduce a plant’s solid waste by over 80 % while lowering caustic chemical (and neutralisation) costs by over 70 %. The removed alumina crystals may be used in a variety of alumina substitutes.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission
Cleaning and regeneration of phosphate solutions
Spent phosphate solution is filtered; the concentrations in metallic ions and the pH are adjusted.
The regenerated phosphate solution is re-used. Reduces the consumption of chemicals, reduces water and sludge releases.
Application especially in continuous electroplanting lines.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 319
Crystallisation of carbonates and metal sulphates
Technology by which interfering salts can be separated selectively from a process solution. These salts are formed by the dissolving of metals or metal oxides (pickling) or by unwanted reactions (oxidation of cyanide to carbonate). By cooling down a solution to <5 °C, the solubility of most of the salts is reduced. Only certain salts in the selected salt mixture crystallise with the cooling down of a solution, while the remaining salts stay in the solution. Simple systems can be utilised, where the solution is pumped to a tank that can be cooled naturally during a winter shut-down period. This also enables other maintenance to be carried such as checking tank liner integrity, removing broken jigs and dropped workpieces.
In Germany, approximately 10 % of installations have this technique installed.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 311
Electrodialysis
- is used both for the concentration of diluted solutions and for the demineralisation of water.
- the electrical dialysis is a diaphragm procedure, in which an electrical field forces material transport. Anions and cations are removed from solutions with an applied electric field in cells with alternating anion- and cation-permeable membranes.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 259, p. 309
Electrolysis – purification of process solutions
Some metallic contaminants can be removed selectively from electrolytes at low current densities from 0.05 to 0.3 A/dm². The efficiency of this selective cleaning can be enhanced with increased electrolyte throughput.
Extension of process solution life, however, not only unwanted metals, but also unused organic additives can be removed. Therefore electrolytic purification may be reduced to a minimum or compensatory additions of organic process materials may be necessary.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 316
Electrolysis – reoxidation of breakdown products
Chromium/sulphuric acid pickling of ABS plastic items oxidises and dissolves the butadiene component of the substrate, simultaneously generating trivalent chromium. Both the organic decomposition product and the trivalent chromium will disturb the process if a tolerable concentration level is exceeded. It is possible to oxidise trivalent chromium without a membrane but with adequate anodic and cathodic density conditions. Ceramic membrane electrolysis is the more reliable means to continuously regenerate process solutions.
Achieved benefits: Extension of the process solution life.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 317
Electrolytic chromium plating - closed loop electroplating
Hexavalent Cr(VI) plating can be operated as closed loop for chromium. This is achieved by a combination of cascade rinsing and an evaporator to ensure that the rinsing water is in equilibrium with the evaporation. Ion exchange is used to remove accumulated impurities. There are no discharges of Cr (VI) or other materials from the process to waste water. This minimises the capital cost of treatment and the use of chemicals and energy in treatment. Chromic acid and other components are recycled in the process.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 264
Evaporation using additional energy with an evaporator
Usually the evaporators used in electroplating shops are equipped with energy recovery (vacuum evaporators with vapour consolidating or heat pump) and need approximately 150 – 200 kWh per cubic metre of evaporated water. Evaporators are increasingly used for electrolyte feedback from rinsing waters. The complete closing of material cycles with an evaporator enabling the complete feed back of rinse-water for certain process stages is achievable.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 256
Extension of the service life of pickling solutions by diffusion dialysis
Reduction in fresh acid, waste acid treatment or disposal costs:
Diffusion dialysis separates acid from its metal contaminants via an acid concentration gradient between two solution compartments (contaminated acid and deionised water) that are divided by an anion exchange membrane. Acid is diffused across the membrane into the deionised water whereas metals are blocked due to their charge and the selectivity of the membrane. A key difference between diffusion dialysis and other membrane technologies such as electrodialysis or reverse osmosis is that diffusion dialysis does not employ an electrical potential or pressure across the membrane. Rather, the transport of acid is caused by the difference in acid concentration on either side of the membrane. As such, the energy requirements for this technology are low.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 330
Filtration of process solutions
Particles suspended in electrolytes can generate negative effects on the layer quality (in particular by inclusion of the particles into the layer). The filtration of process solutions is used to remove particles (e.g. splinters or dirt), which were introduced by the workpieces/substrate, anode mud, dust from the air or the insoluble compounds developed during the process (such as metal hydroxides). In order to guarantee a continuous removal of the solids, the filter is operated in a bypass to the process tank.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 308
Increasing drag-out recovery rate and closing the loop
Where the quantity of water needed for appropriate rinsing (to achieve process control and product quality) exceeds evaporation losses, and recovery rates>90 % are expected, the amount of water in the drag-out recovery system has to be decreased. This is achieved by a combination of techniques.In some cases, drag-out can be recovered until the loop can be closed for process chemicals by applying a suitable combination of techniques.
Closing the loop achieves a high raw material utilisation rate and in particular can:
- reduce the use (and therefore cost) of raw materials and water
- reduce the need for end-of-pipe waste water treatment (e.g. removing nickel from contact with effluent containing cyanide)
- reduce overall energy usage when used in conjunction with evaporation to replace cooling systems
- as a point-source treatment technique, achieve low emission limit values
- reduce the use of chemicals for treating the recovered materials that would otherwise be discharged in the waste water
- reduce the loss of conservative materials where used
Increasing drag-out recovery and closing the loop require techniques to:
- reduce dt
- reduce rinse-water (such as by cascade rinsing and/or sprays) with drag-out recovery
- concentrate the returning drag-out or receiving solutions, such as by ion exchange, membrane techniques, or evaporation. The water removed during concentration (such as from evaporation) can often be recycled back into the rinse.
Examples of techniques for this purpose are, for example:
- addition of an eco rinse tank
- evaporation using surplus internal energy
- evaporation using additional energy (and in some cases, low pressure)
- electrodialysis
- reverse osmosis.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 253
Maintenance of degreasing solutions
Cascade (multiple) use of degreasing solutions
- Significant reduction in electrolytes and water consumptions within the processes and hence a reduction in the quantity of waste water volumes.
Simple methods - filtration with cellulose filters, mechanical separation by skimmers, gravity oil separators
- extendthe working life of decreasing solutions by removing oil
Static separator for degreasing baths
- large fall of the COD in the effluents, by 50 % in some cases; significant reduction in dumping of used solutions: in most cases reduction of between 50 and 70 %; reduction of detergent purchases by 50 %
Biological degreasing regeneration
- runs at more neutral pH, with lower operating temperatures of around 45 °; reduced use of process chemicals as the solution rarely needs replacement; reduction in use of hazardous chemicals in the workplace; reduced usage of neutralising chemicals when discharging used process solution and lower impact of surfactants on effluent treatment; lower evaporation losses therefore less need to extract water vapour
Centrifuging of degreasing baths
- reduces the discarding of used baths contaminated with oil and solids; pollution in the degreasing bath is maintained at a constant low level, reducing the level of drag-out and minimising the use and pollution of rinsing water; oil is recovered and concentrated at the output of the separator for possible recovery. Sludges are collected separately, reduction of the draining frequencies (between 30 and 80 %) according to the size, the production and many other parameters relative to the installation, with less heating and downtime required, no loss of surface active agent
Membrane filtration of emulsifying degreasers (micro- or ultrafiltration)
- reduced chemical and energy consumption in degreasing heavily contaminated workpieces or substrates; increase of the degreasing bath lifetime (up to 10 times); reduction of detergent consumption by 50 %; high reduction in pollution, reducing COD between 30 and 70 % according to water agency data; less discarding of the used baths (usually with oil levels between 10 and 15 g/l)
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 320
Measures for decreasing pickling acid consumption
A three-stage cascade hydrochloric acid system running at 0.5 l/min is being used successfully to remove hardening scale from parts prior to plating.
The system is identical to a cascade water rinse system, but uses 32 % hydrochloric pickling acid instead of water.
Extending chemical process life. A three-stage cascade system has reduced chemical usage by 50 %. A smaller, continuous flow of hydrochloric acid is more readily treated in a typical waste water treatment plant, removing the problems caused by batch discharges of acid to treatment.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 329
Membrane electrolysis for chromium solution maintenance
Membrane electrolysis uses one or more ion-selective membranes to separate electrolyte solutions within an electrolysis cell. The membranes are ion-permeable and selective.
Membrane electrolysis can regenerate process solutions through two primary mechanisms:
- Selective transfer of ions from the process solution, across the membrane, into an electrolyte solution and
- Regenerating oxidation states/ionic forms of key constituents in the process solution through electrode electrochemical reactions.
An electrolytic cell technique with fluidised bed technology and used in conjunction with semipermeable membranes extends the life of a hexavalent solution by 300 to 400 %.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 318
Multiple rinse techniques
Multiple stage rinsing is particularly suitable to achieve a high rinsing rate with a small amount of rinsing water. For example, in cascade rinsing, the water flows in the opposite direction to the workpieces. A smaller rinsing quantity of water can be achieved by the selection of the correct rinsing system. The effect of water saving decreases with an increasing number of rinsing stages. However, the volume of water required decreases to the point where direct make up for water losses from process solutions at ambient temperatures can be considered. The achievable recovery rate is, at a given volume of evaporation, directly related to the concentration of process chemicals in the first rinse station. Closing the loop for a process requires the water returned to the process solution from the first rinse station to be brought into balance with the water lost in evaporation and drag-out. Process solutions operated at higher temperatures and with multi-stage rinsing offer possibilities for this. By the introduction of multistage rinsing systems partly combined with a rinsing water recycling system and other techniques and decreases of waste water of up to 90 % can be obtained. One coil coating plant reports a reduction of 30 m3 per hour.
- Multiple stage counterflow rinse
- Multiple static rinse
- Dual static rinse followed by final flow rinse with recirculated water
- Multi-cascade rinsing with limited process line space (cascades are external to the process line due to limited space; in the treatment line there is only one rinsing tank per process step. Each rinse tank is connected to several external tanks which work as rinsing stages according to the cascade principle. The workpieces or substrates are brought into the rinsing tank and rinsed successively with the water from the individual rinsing stage tanks, becoming progressively cleaner. Rinsing can be by sprays or filling the tank to immerse the workpieces or substrates)
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 245, p. 252
Recovery of precious metals from rinses
Electrolytic recovery
- recovery of metals for re-use; reduction of metals in drag-out and their consequent decrease in effluent concentrations; in the electrolytic separation of metal solutions containing cyanide, the anodically oxidative destruction of the cyanide takes place in parallel to the metal winning
Ion exchange – recovery of precious metals from rinses
- Precious metals in concentrated solutions are usually recovered electrolytically, while more dilute solutions, sometimes of no more than a few mg/litre, are treated by adsorption of the metal content on ion exchange resins .Ion exchange provides only a concentration of the metal in the resin, the subsequent recovery being possible by incineration of the resin or by releasing the metal in dissolved form, but at higher concentration. The final metal recovery by incineration is in an oxygen-rich atmosphere at 500 – 600 °C; metals are found with the residual ash. Recovery is about 95 % efficient.
- The hexavalent chromium in chromating solutions is exhausted after a certain time. The solutions also dissolve and accumulate zinc and other metals and eventually lose their workability, and must then be rejected and renewed.Regeneration is usually only cost-effective with relatively concentrated and expensive solutions, for example, black chromating solutions containing silver.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 336
Regeneration and re-use/recycling of rinsing water
Spent rinse-water can be regenerated and this can lead to savings in water consumption and will reduce to amount of waste water to be treated, reducing the waste water treatment costs for capital investment, energy usage and chemicals.
- By feeding the rinsing water through cation and/or anion exchangers, the cations become exchanged for H+, and the anions for OH, and water of a quality approaching demineralised water is achieved. This is fed back to the rinsing system.
- Regeneration by reverse osmosis: used to concentrate rinse-waters and recover materials, treat waste waters and incoming or recycled water. Reverse osmosis uses a hydrostatic pressure gradient across a semi-permeable membrane to separate water from a solution of salts. The pressure applied exceeds the osmotic pressure of the feed solution causing water to flow from the concentrated solution to the more dilute solution: the reverse of the natural osmotic diffusion. Dissolved solids are rejected by the membrane surface. Many multi-charged ions can be rejected at rates exceeding 99 %. Singlecharged ions typically have rejection rates in the range of 90 - 96 %.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 243, p. 261
Retardation (acid resin sorbtion)
Retardation (or acid resin sorption) is an ion exchange separation technique used primarily for the regeneration of acids (e.g. pickling acids and etchants such as in anodising). A high concentration solution containing metal (or acid salt) is pumped upstream through an ion exchange resin, where the major proportion of the acid anions penetrate into the resin of an anion exchanger while the metal cations are excluded by electrostatic repulsion, and pass through. In the second step, water is pumped downstream through the resin; the acid is set free again. The recovered acid can be re-used. A depletion rate of between 40 and 60 % can be achieved, depending on the type of acid and metal.
Can be used on
- sulphuric acid anodising baths for aluminium
- sulphuric or nitric acid pickling, etching, or brightening baths for copper or brass
- nitric/hydrofluoric acid pickling baths used for processing stainless steel
- phosphoric and/or sulphuric acid baths for stainless steel or aluminium electropolishing
- cation ion exchange acid regenerated solutions
- sulphuric or hydrochloric acid pickling baths for steel and galvanized steel.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 309
Projects
Change of existing galvanization plants to meterials loss minimized process technique at simultaneous costs reduction: Change of a hot-dip galvanising (TV 16)
- optimization of the degreaser and development of a regenerator for the discharge of oil/grease from the degreaser solution for unlimited bath life: cost reduction of about 6,000 EUR/year
- installation of a cross-flow/counter flow heat recovery plant and further energy saving measures: energy savings of about 150-170 kWh (= 30-35%)
- measures for savings in materials through "Partial Automation Galvanizing Kettle", "Application of New Zinc Alloys", "Installation of a Zinc Ash Treatment Plant", "Application of a Vibration Hard Zinc Grab", and other peripheral individual measures: savings in zinc compared to the initial situation of about 17% as well as additionally about 12,000 €/year due to other effects
- the application of the ion exchange process enables the zinc-elimination from the mixed acid pickles; with this, logistical advantages (higher plant productivity) are utilized and internal cycles are being closed: savings potential of about 50,000 EUR/year
Change of existing galvanizing plants to material loss minimised process technology at simultaneous cost reductionConversion of existing electroplating plants to a process technology with minimum material losses and simultaneous cost reduction
- avoidance of material losses through closure of internal material cycles
- reduction of the amount of electroplating slurries and waste water volumes, and thus of costs
- low silver loss through consistent recycling from all process solutions and rinsing
Changing existing galvanization plants to materials loss minimized technique at simultaneous reduction: Galvanotechnik Breitungen
- reduction of material losses in the metallization of plastics (layer series copper/nickel/chromium) through the change of process and plant technology, the optimization of volume flows and material flow control measures
- cost reduction through the development of internal material and water cycles
- material cycles with recirculation rates of about 90% through the use of evaporators and regenerators
- reduction of material consumption (chemicals, water) and the amount of waste water and waste
Completion of the material cycle in procedures for the removal of materials in process solutions: membrane application technology and testing in a hot-dip galvanising plant
- electrodialysis for rinsing water treatment
- energy requirement for the treatment of 1.4 m³/h is 0.5 kW/h
Construction, testing and optimization of a low-waste electroplating plant operated without waste water to be drained
- waste water-free operation of the plant due to recycling of production chemicals and recirculation of auxiliary production materials
- the small residual amount of production waste water is vaporized in an energetically optimized evaporation plant; in this process, distilled water is extracted which is then returned to the process in the form of rinsing water
- reduction of residues: electroplating slurries by 75%, phosphating sludges by about 40%; acids, alkaline solutions, chromating solutions and cyanidic solutions were completely eliminated from the residue balance
- the operating costs, which are slightly higher than those of a conventional plant, are accompanied by considerable savings in disposal costs, procurement costs for raw materials (input of metal and chemicals) and energy costs.
Development and testing of a process technique for the regeneration of electroplated electrolytes – industrial framework
- nearly unlimited life-time of the electrolytes due to waste-water free process control with few waste products
- regenerative adsorbing polymers remove organic contaminants from the plating bath without major losses
Development of an environmentally friendly technology for metal chemistry and electroplating
- minimization of the carry-over of process solutions in rinsing processes by means of new geometries and surfaces of transport baskets
- no modification of existing plant required
- regeneration method for a particular tin-nickel electrolyte enabling a nearly complete closure of the material cycle in this sub-process
- complete separation of recyclables and decomposition products (contaminants) through evaporation
Development, testing and optimisation of process technology for the pickling/etching of composite metals with the minimal loss of material
- regeneration of the hydrochloric acid during etching of composite metal
- cost savings of about 25,000 €/year, since the consumption of process and neutralization chemicals as well as the amount of the accumulated slurry is being reduced by 90%
Environmental Plating of Steel: I. Replacement of Cadmium by Zinc/Iron, II. Recycling of Electrolyte-Enriched Rinsing Water
- due to a three-stage reverse osmosis plant integrated in the rinsing cycle which has a retention capacity of over 99%, the electrolyte-enriched rinsing water is separated from the electrolyte and returned into the electrolyte baths
- cadmium was replaced by the material combination of zinc/iron
- zinc/iron coating and acid galvanizing are carried out in closed material cycles
- residues are avoided or unavoidable residues occur in recyclable form
BUT: significant deterioration of product quality --> thus selected process cannot be translated into practice!
To complete Project DescriptionPickling and roasting of semi-finished components of copper and cupro-nickel composition without the use of nitric acid and chromic acid
- replacement of chromium acid and nitric acid as pickling solutions
- continuous regeneration of the pickling solution
- copper recovery by means of electrolysis resp. membrane electrolysis
- waste water-free process
Planning, construction and testing of a demonstration electroplating facility for nickel- and chromium-plating with the complete recycling of nickel and chromium
- complete recovery of problematic heavy metals in galvanic nickel plating and chroming
- extension of service life of electrolytes through removal of undesirable decomposition products and recirculation of cleaned electrolytes
- minimization of rinsing water input in all process steps through immersion rinsing cascades with clocked spray rinsing and multiple use of the rinsing water in the case of three-stage pretreatment
Reorganization of galvanic plants to material loss minimised process techniques with simultaneous reduction of costs: electroplating repairs
- cost savings of 80% through production-integrated optimization measures (in particular in electroplating shops specialized in aircraft repairs)
- a considerable part of the savings is realized by the recovery of potassium silver cyanide by means of vacuum evaporation
Reorganization of galvanic plants to material loss minimised process techniques with simultaneous reduction of costs: rack for automatic metal surface finishing
- increased recirculation rate and significant reduction of the consumption of process chemicals and chemicals for waste water treatment through optimized rinsing technology in connection with an ion exchanger recirculation plant
- closed internal material cycle without peripheral concentrator and regenerator for the subsystem of chromium
Reorganization of galvanic plants to material loss minimised process techniques with simultaneous reduction of costs: technologically- and scientifically-oriented operation
- introduction of material-loss minimized process technology in a company mainly specialized in high-quality coatings
- the closing of internal material cycles enabled a considerable reduction of water consumption and waste water formation
Sewage and waste reduced electroplating plant through water recycling and producing of secondary raw materials
- when degreasing, additional pre-degreasing is carried out exclusively with hot water in order to save detergents
- clocked spray rinsing and recirculation enabled the reduction of waste water to 20-30% in comparison to conventional plants
- regeneration of cleaning baths by means of micro-filtration and reverse osmosis
- deoxidation baths are being concentrated through reverse osmosis and added to the pickling bath
- exhausted pickles are being processed through the separation of the contained metals (iron and zinc) by means of ion exchangers
- non-recoverable metals (iron, chromium and zinc of chromating baths) are being selectively separated through gradual precipitation at different pH-values and are then processed
- waste waters from phosphating are separately collected and treated; residues are fed to zinc slurry treatment
- process solutions from demetallization are separately electrolytically processed
- the zinc and nickel containing solutions of the electrolytical metal deposition are recirculated in an almost closed cycle
- energy-saving concentration of the rinsing waters to be recycled is carried out by means of reverse osmosis and evaporation
Videos
Final decision in the anodizing bath
By clicking the Play button you agree that your data will be transmitted to YouTube and that you have read the privacy policy.
Cleaning
Best Available Techniques
Choice of manufacturing process prior to surface treatment
- a different pressing oil, which may become pressed into the substrate micro-sturcture, might not respond to normal degreasing processes
- reduction of oil and grease applied in the mechanical production area via: use of volatile lubricants, employment of minimal quantity cool lubrication, dripping off and/or centrifuging the workpieces, pre-cleaning the workpieces at the point of production, shortening the storage time, drilling with compressed air cooling, use of applied plastic film lubricants in pressing
- a therefore optimised cleaning reduces reworking
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 200, p. 215
Drag-in reduction
Drag-in can contaminate a process solution if is there is insufficient rinsing after the previous processes. Drag-in of clean rinse-water can significantly dilute a process solution. Drag in can be minimised by using an eco-rinse (or pre-dip), see Section 4.7.4, or by removing as much rinse-water as possible, such as by air knives or wiper rollers for sheet or coil substrates. The effects can also be minimised by using compatible chemical systems Drag-in reduction leads to extending process solution life, improving process quality and reducing material costs in make up chemicals.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 229
Eco rinse or pre-dipping
Some drag-out from process solutions working at (but not limited to) ambient temperature can be recovered through a single rinse station in which the workload is dipped before and after being processed. The eco rinse station (or pre-dip) can be made up with diluted process solution from the very beginning or filled with deionised water only. In this case it will take some time until the final equilibrium concentration of 0.5 C0 (50 %) will be reached. The solution only has to be changed when the tank itself and/or the tank walls have to be cleaned. During normal operation, no water has to be added assuming that drag-in is equivalent to dragout. Drag-out recovery rate (jig and barrel plating) is approximately 50 %.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 239
Maintenance of degreasing solutions
Cascade (multiple) use of degreasing solutions
- Significant reduction in electrolytes and water consumptions within the processes and hence a reduction in the quantity of waste water volumes.
Simple methods - filtration with cellulose filters, mechanical separation by skimmers, gravity oil separators
- extendthe working life of decreasing solutions by removing oil
Static separator for degreasing baths
- large fall of the COD in the effluents, by 50 % in some cases; significant reduction in dumping of used solutions: in most cases reduction of between 50 and 70 %; reduction of detergent purchases by 50 %
Biological degreasing regeneration
- runs at more neutral pH, with lower operating temperatures of around 45 °; reduced use of process chemicals as the solution rarely needs replacement; reduction in use of hazardous chemicals in the workplace; reduced usage of neutralising chemicals when discharging used process solution and lower impact of surfactants on effluent treatment; lower evaporation losses therefore less need to extract water vapour
Centrifuging of degreasing baths
- reduces the discarding of used baths contaminated with oil and solids; pollution in the degreasing bath is maintained at a constant low level, reducing the level of drag-out and minimising the use and pollution of rinsing water; oil is recovered and concentrated at the output of the separator for possible recovery. Sludges are collected separately, reduction of the draining frequencies (between 30 and 80 %) according to the size, the production and many other parameters relative to the installation, with less heating and downtime required, no loss of surface active agent
Membrane filtration of emulsifying degreasers (micro- or ultrafiltration)
- reduced chemical and energy consumption in degreasing heavily contaminated workpieces or substrates; increase of the degreasing bath lifetime (up to 10 times); reduction of detergent consumption by 50 %; high reduction in pollution, reducing COD between 30 and 70 % according to water agency data; less discarding of the used baths (usually with oil levels between 10 and 15 g/l)
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 320
Substitution and choices for degreasing
Mechanical pre-cleaning - centrifuging
- Excessive oil and grease can be removed mechanically, i.e. by centrifuging or by air knife before other forms of degreasing, such as chemical or solvent. This extends the life of degreasing solution and leads to savings in chemicals and other inputs for degreasing and a reduction in wastes produced.
Solvent degreasing
- used for high specification work, e.g. some aerospace or military applications and where water-based treatments can damage the surface being treated
Chemical aqueous (soak) degreasing
- offers well proven alternatives to solvent-based systems in almost all cases. The acids and alkalis used are simple and easy to treat in typical waste water treatment plants, unless strong chelating agents are present.
Weak emulsion degreasing
- this is a variation of chemical aqueous degreasing, using a more easily maintained solution. Surface-active agents used in weak emulsion degreasing solutions are developed chemically so they do not form a stable emulsion with the removed oils and greases. The degreasing tanks are drained to a holding tank (usually for a group of degreasing tanks) for the removal of floating oils and sediments. Weak emulsion cleaning solution separates by itself, so that simple mechanical systems (skimmers) can be used for the removal of the oil. By the continuous removal of contamination via the holding tank and feedback of the cleaned degreasing solutions in the bath, a high service lifetime is achieved.
Biological degreasing
- this is a maintenance technique for weak alkali degreasing baths that overcome their short lifetime by constant bypass regeneration
Dry ice
- Removal of oil, grease and particles, paint, etc. without the use of solvents. Dry waste containing only removed components.
Ultrasonic cleaning
- Ultrasonic cleaning uses high frequency sound waves to improve the cleaning efficiency of aqueous, semi-aqueous and solvent cleaners. By generating zones of high and low pressure in the liquid, the sound waves create microscopic vacuum bubbles that implode when the sound wave moves and the zone changes from negative to positive pressure: this is called cavitation. If this occurs at the surface to be cleaned, the pressure cycles lead to local impacts, resulting in a mechanical action at the surface. Theoretically, localised pressures of >1000 bar are generated, dislodging grease and dirt. Cleaning agents make this a viable process in aqueous solutions. --> More effective cleaning with less hazardous chemicals, when using aqueous solutions.
Electrolytic cleaning with pH control
- The degreasing solution is continuously monitored using pH to measure its effectiveness and to control the addition of new solution. This minimises the use of degreasing solution and the amount of waste solution requiring treatment. Reduces the volume of waste water and sludge from the waste water treatment plant.
High performance degreasing systems
- For high quality cleaning, aqueous systems are used which can be supplemented by electrolytic action. High quality cleaning is essential with modern process solutions such as acid zinc, etc. In the case of strongly oiled parts, multistage degreasing can be advantageous. For the first stage, a hot water pre-degreasing or an unstable emulsion cleaning solution is used. For a second stage, a more strongly emulsifying cleaning solution is used. Also the combination of any two degreasing baths in sequence with the second, cleaner bath being used to replenish or replace the first, dirtier bath extends the service life of the degreasing solutions considerably. Extension of life of degreasing solutions and reduction or reworking.
hot water
- hot water (80 - 90 °C) without chemicals can remove the majority of oil and grease. This method is used mainly in the automotive industry when cleaning pressed sheet steel. Using a high pressure water jet further improves the effect.
hand wiping
- large and/or high value components can be cleaned manually with cloth or paper wipers.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 291
Projects
Change of existing Electroplating plants to a process technique of minimised material losses and reduced costs: comparison of different biodegradation procedures implemented in order to regenerate degreasing solutions (Part 1)
- significant extension of the service life of the degreasing bath
- introduction of a cascade pickling system: significantly higher pickle quality in comparison to the initial condition and considerable increase in the utilization rate of the pickling acid
Conversion of an electroplating shop to environmentally friendly pickling baths
- reduction of the fresh acid demand, further additives in the pickling baths and demand for chemicals for waste water treatment
- reduction of spent acid as well as of waste and waste water production
- minimization of costs for fresh acid, treatment chemicals, treatment time and expenses for spent acid, downtimes for new bath preparations etc.
Development, testing and optimisation of process technology for the pickling/etching of composite metals with the minimal loss of material
- regeneration of the hydrochloric acid during etching of composite metal
- cost savings of about 25,000 €/year, since the consumption of process and neutralization chemicals as well as the amount of the accumulated slurry is being reduced by 90%
Pickling and roasting of semi-finished components of copper and cupro-nickel composition without the use of nitric acid and chromic acid
- replacement of chromium acid and nitric acid as pickling solutions
- continuous regeneration of the pickling solution
- copper recovery by means of electrolysis resp. membrane electrolysis
- waste water-free process
Sewage and waste reduced electroplating plant through water recycling and producing of secondary raw materials
- when degreasing, additional pre-degreasing is carried out exclusively with hot water in order to save detergents
- clocked spray rinsing and recirculation enabled the reduction of waste water to 20-30% in comparison to conventional plants
- regeneration of cleaning baths by means of micro-filtration and reverse osmosis
- deoxidation baths are being concentrated through reverse osmosis and added to the pickling bath
- exhausted pickles are being processed through the separation of the contained metals (iron and zinc) by means of ion exchangers
- non-recoverable metals (iron, chromium and zinc of chromating baths) are being selectively separated through gradual precipitation at different pH-values and are then processed
- waste waters from phosphating are separately collected and treated; residues are fed to zinc slurry treatment
- process solutions from demetallization are separately electrolytically processed
- the zinc and nickel containing solutions of the electrolytical metal deposition are recirculated in an almost closed cycle
- energy-saving concentration of the rinsing waters to be recycled is carried out by means of reverse osmosis and evaporation
Strip cleaning with brilliant results
- environmentally friendly detergents and technological innovations to reduce the consumption of large amounts of water and chemicals
- higher cleaning efficiency at lower environmental pollution through novel treatment process in which the cleaning medium is being recirculated
Substitution of galvanic processes using secondary plasma flame coating
- Ion source with deflectable beam for secondary ion beam coating as an alternative to electroplating
- no toxic waste water or acid or base vapour in ion beam coating due to the elimination of chemical pre-cleaning steps and subsequent treatment
- the coating rates of chromium are higher than those of electroplating
- the ion beam coating method influences the structure of the deposited layers to a far greater extent and prevents columnar crystal formation
Electroplating
Best Available Techniques
Agitation of process solutions
Agitation of process solutions to keep a consistent solution concentration
throughout the vat and prevents the build-up of gas bubbles and contaminants at the workpiece or substrate surface, giving uneven finishes, pitting, etc.
The options are:
- compressed air through nozzles
- hydraulic turbulence
- agitation of the workpieces by moving the flight bars or rods by cams or motors.
- In barrel treatments, sufficient agitation is usually achieved by the turning of the barrels and movement of the workpieces within.
- low pressure air agitation systems in:
- solutions where the air assists cooling by evaporation particularly when used with materials recovery
- anodising
- other processes requiring high turbulence to achieve high quality
- solutions requiring oxidation of additives
- where it is necessary to remove reactive gases (such as hydrogen).
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 216, p. 395
Control of concentration of process chemicals
Process solutions become increasingly ineffective when the working concentration of certain process chemicals drops below specification. By topping up of the consumed process chemicals the service lifetime of a solution can be extended, see Section 4.1.2. SPC controls are often used and/or other production management systems A key problem remains that some production operators tendency to add more material than is necessary. Where possible, automated dosing is the best option for accuracy and reliability, and allows regular additions and avoids swings in concentration. This may be actuated on a time, temperature, flowrate, or other control basis, such as pH or rH, etc. Existing processes can be optimised by suppliers and/or in-house expertise to reduce the concentration of chemicals, particularly those with significant environmental or health effects.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 267
Cooling
- Using closed cooling systems saves water.
- prevent over-cooling by optimising the process solution composition and working temperature range. Monitor temperature of processes and control within these optimised process ranges.
- use closed refrigerated cooling system, for new or replacement cooling systems
- remove excess energy from process solutions by evaporation where there is a need to reduce the solution volume for make-up chemicals or evaporation can be combined with cascade and/or reduced water rinsing systems to minimise water and materials discharges from the process
- install an evaporator system in preference to a cooling system where the energy balance calculation shows a lower energy requirement for forced evaporation than for additional cooling and the solution chemistry is stable,
- design, locate and maintain open cooling systems to prevent the formation and transmission of legionella
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 233 + p. 396
Different electrode yields
A higher anodic yield leads to an increase of the metal ion concentration. This can be found with certain electrolytes, such as nickel and zinc solutions. are discussed under Applicability, below:
- where solution electrochemistry allows, use insoluble anodes with external dissolution of the metal and controlled solution strength
- replace some of the soluble anodes by membrane anodes with an extra current circuit
- special insoluble anodes that allow the concentration of the solution to balanced
- run workpieces or substrates requiring higher thickness coatings
- ‘plating out’ on steel sheet
- removing anodes.
Achieved environmental benefits:
- Minimisation of energy usage and waste of process metal in drag-over.
- Reduction of plating over the required specification thickness.
- Reduction in environmental effects from reworking due to problems with over-plating.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 269
Drag-in reduction
Drag-in can contaminate a process solution if is there is insufficient rinsing after the previous processes. Drag-in of clean rinse-water can significantly dilute a process solution. Drag in can be minimised by using an eco-rinse (or pre-dip), see Section 4.7.4, or by removing as much rinse-water as possible, such as by air knives or wiper rollers for sheet or coil substrates. The effects can also be minimised by using compatible chemical systems Drag-in reduction leads to extending process solution life, improving process quality and reducing material costs in make up chemicals.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 229
Drag-out reduction
A reduction of drag-out is an effective primary measure for:
- minimising losses of chemicals in rinses
- reducing the amount of rinsing required
- reducing raw material costs
- reducing quality and maintenance problems with subsequent processes
- reducing environmental problems associated with rinsing waters.
Drag-out depends on a large variety of parameters and a reduction of this key step with many impacts on the environment and the process can only be achieved by close co-operation of all personnel involved. For this reason a thorough understanding of the complex interrelations of many parameters is needed by the operational staff to improve the situation successfully, see issues such as training in environmental management systems.
- Measures for drag-out reduction: The use of compatible chemicals (e.g. the use of the same acid in pickling or activating the surface prior to an acid-based plating process) reduces the consequences of chemical drag-over to the subsequent process.
- jig (rack) processing: Arrange the largest surfaces of the workpieces in a vertical position at the jigs (racks) allows the adhering solution to run down to the bottom edge of the workpieces. When lifted out of the process solution, the jigs may be tilted in such a way that large droplets can be formed faster and drip down from the lowest point of the suspended articles. Allow sufficient drainage time above the process tank to let the adhering liquid to cohere and form droplets which will drip from the articles By slow withdrawal of the jigs from the process solution, the drag-out volume can be decreased considerably. Cup-shaped recesses are normally avoided where possible, and cup-shaped components are jigged cup-side down on the incline so process solution is not carried into the rinse-water. In some cases, arrangements can be made by dialogue with customers for components with high drag-out retention, such as cup-shaped components, to be manufactured with drainage holes. Dripping of process solution on other articles arranged lower on the jig is normally addressed by suitable positioning of the workpieces. Drag-out by jigs can be reduced by inclining supporting arms to avoid horizontal surfaces from which the adherent solution cannot easily run off. A normal inspection and maintenance task is to check the insulation coating of the jigs to ensure smooth surfaces, with no fissures or cracks in damaged insulation to trap and retain solution. It is good practice to regularly inspect jigs for defective insulation so they can be identified for replacement or repair.
- barrel processing: The plastic material of the barrel normally has a smooth surface and is inspected for worn areas and the formation of recesses or bulges around the holes. The bores of holes in the panels usually have a sufficient cross-section to minimise capillary effects, and the thickness of the panels of the cylinder is just thick enough to meet the mechanical strength requirements. The total proportion of the body of the barrel that is perforated is usually as high as possible to allow the drag-out to drop back easily into the process tank. This also improves the efficiency of the whole plating process by allowing easier solution access and decreasing voltage drop. A further reduction of drag-out can be attained by intermittent rotation of the barrel above the process tank while draining (such as rotating for about 90 degrees, stopping for at least 10 seconds, next sequence of intermittent rotation, etc.). More reduction of drag-out can been achieved by the application of draining ledges within the barrels to allow the draining liquid to flow together and to drain out of the rotating barrel. Drag-out can be reduced dramatically by blowing excess solution out of the barrel while draining over the bath. With hot baths, the barrels can be rinsed with water or sprayed, although for barrels, sparging is more effective: sparging is where pipe is constructed within the barrel and runs rinse-water inside the barrels and through the workpieces. In a barrel, the workpieces usually lie with the main surfaces horizontal. To achieve better draining, inclined lifting of the barrels from the tanks can be considered. The suspension and hoisting systems may be adapted to this requirement. The application of mesh plugs instead of holes has proven successful, by reducing the length of the bores in the panels of the cylinder body of the barrel. The drag-out can be decreased, and the voltage drop at the perforation is effectively reduced.
- Properties of process solutions: Drag-out can be reduced by raising the temperature of the process solution which normally lowers the viscosity of the solution. Lowering the concentrations of the process solutions will effectively reduce the drag-out, by lowering the amount of material contained in the dragged-out solution, as well as reducing surface tension and viscosity of normal ionic solutions. The addition of wetting agents to the process solution reduces the drag-out by reducing surface tension. To avoid excessively increased concentrations, the process solution may be controlled to a constant composition during regeneration and maintenance. This, and the selection of appropriate process solutions, is an important step in the reduction of drag-out.
- Transition from drag-out draining to rinsing: When jigs (racks) or barrels are being removed from a tank of heated solution, it is good practice to drench it with a fog spray while it is still over the processing tank. This achieves a reduction in drag-out loss, and the water used compensates for evaporation. This treatment can be combined with a pre-rinse, returning water from the first static rinse to the process solution. For removing solution adhering to, or trapped in, recesses, combined water and air jets may be used above the process tank and within an empty tank, respectively.
Electrolytic chromium plating - closed loop electroplating
Hexavalent Cr(VI) plating can be operated as closed loop for chromium. This is achieved by a combination of cascade rinsing and an evaporator to ensure that the rinsing water is in equilibrium with the evaporation. Ion exchange is used to remove accumulated impurities. There are no discharges of Cr (VI) or other materials from the process to waste water. This minimises the capital cost of treatment and the use of chemicals and energy in treatment. Chromic acid and other components are recycled in the process.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 264
Filtration of process solutions
Particles suspended in electrolytes can generate negative effects on the layer quality (in particular by inclusion of the particles into the layer). The filtration of process solutions is used to remove particles (e.g. splinters or dirt), which were introduced by the workpieces/substrate, anode mud, dust from the air or the insoluble compounds developed during the process (such as metal hydroxides). In order to guarantee a continuous removal of the solids, the filter is operated in a bypass to the process tank.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 308
Heating of process solutions / Reducing heating losses
- Process solutions may be heated by energy coming from process steps generating energy.
- Water from the cooling circuit of various process solutions may be used to heat lower temperature solutions, incoming air, etc.
- Alternatively, the hot cooling water is collected in a central tank and cooled through a suitable heat pump. The gain in energy may be used to heat process solutions with process temperatures up to 65 °C, or to heat up water for other purposes.
- Using insulated tanks and floating spheres to insulate the solution surface
- Reducing the operating temperature of solutions
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 222, p. 396
Regeneration and re-use/recycling of rinsing water
Spent rinse-water can be regenerated and this can lead to savings in water consumption and will reduce to amount of waste water to be treated, reducing the waste water treatment costs for capital investment, energy usage and chemicals.
- By feeding the rinsing water through cation and/or anion exchangers, the cations become exchanged for H+, and the anions for OH, and water of a quality approaching demineralised water is achieved. This is fed back to the rinsing system.
- Regeneration by reverse osmosis: used to concentrate rinse-waters and recover materials, treat waste waters and incoming or recycled water. Reverse osmosis uses a hydrostatic pressure gradient across a semi-permeable membrane to separate water from a solution of salts. The pressure applied exceeds the osmotic pressure of the feed solution causing water to flow from the concentrated solution to the more dilute solution: the reverse of the natural osmotic diffusion. Dissolved solids are rejected by the membrane surface. Many multi-charged ions can be rejected at rates exceeding 99 %. Singlecharged ions typically have rejection rates in the range of 90 - 96 %.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 243, p. 261
Retardation (acid resin sorbtion)
Retardation (or acid resin sorption) is an ion exchange separation technique used primarily for the regeneration of acids (e.g. pickling acids and etchants such as in anodising). A high concentration solution containing metal (or acid salt) is pumped upstream through an ion exchange resin, where the major proportion of the acid anions penetrate into the resin of an anion exchanger while the metal cations are excluded by electrostatic repulsion, and pass through. In the second step, water is pumped downstream through the resin; the acid is set free again. The recovered acid can be re-used. A depletion rate of between 40 and 60 % can be achieved, depending on the type of acid and metal.
Can be used on
- sulphuric acid anodising baths for aluminium
- sulphuric or nitric acid pickling, etching, or brightening baths for copper or brass
- nitric/hydrofluoric acid pickling baths used for processing stainless steel
- phosphoric and/or sulphuric acid baths for stainless steel or aluminium electropolishing
- cation ion exchange acid regenerated solutions
- sulphuric or hydrochloric acid pickling baths for steel and galvanized steel.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 309
Rinsing techniques and drag-out recovery
Eco rinse or pre-dipping:
- Some drag-out from process solutions working at (but not limited to) ambient temperature can be recovered through a single rinse station in which the workload is dipped before and after being processed. The eco rinse station (or pre-dip) can be made up with diluted process solution from the very beginning or filled with deionised water only. In this case it will take some time until the final equilibrium concentration of 0.5 C0 (50 %) will be reached. The solution only has to be changed when the tank itself and/or the tank walls have to be cleaned. During normal operation, no water has to be added assuming that drag-in is equivalent to dragout. Drag-out recovery rate (jig and barrel plating) is approximately 50 %.
Spray rinsing:
- the process solution or in a separate empty tank. SSpraying prior to rinsing (or pre-rinse) above the process bath is an effective method of rinsing. The rinsing water is sprayed onto the workpieces while they are still above the bath surface. For the pre-rinse, the amount of water to be used should equal that dragged-out from the process tank to maintain the water balance. The pre-rinse causes a direct feedback of process solution into the process tank. Spray rinsing in a separate tank acts as a first rinse. The solution can then be recycled back to the process solution in amounts equal to evaporative and drag-out losses. This is a key step in reducing the loss of soluble chemicals from process vats to the environment via rinsing.
Manual or semi-automatic lines:
- are used for small production throughput, or for development work. Controlling water usage, drag-in and drag-out may appear more difficult. To achieve adequate draining time on a manual line, the jig or barrel should be supported on a static rack over the preceding bath.This enables spray rinsing to be carried out directly above the treatment tank to return the drag-out and/or allows draining to be properly timed before immersion in a rinse. In semi-automatic lines, spray rinsing can be also carried manually.
Chemical rinses:
- An accelerated technique to achieve the required cleaning efficiency is obtained by means of chemical rinses called the Lancy process. Here the dragged-out process solution is reacted chemically with the rinsing liquid at the same time. Reduces the effluent treatment capacity required by reducing or eliminating the primary waste water treatment stages. Incorporation of chemical rinsing can reduce the number of rinse stations with a consequent increase in contaminated rinsing.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 239
Substitution by alternative processes
To achieve modern high standards for corrosion prevention, multilayer systems are increasingly used. This enables the operator to offer alternative systems to customers that achieve the same performance criteria, and the potential to move away from more polluting processes. For example:
- electroless nickel for some hard chromium applications
- zinc alloys in place of zinc and chromium passivation, in conjunction with organic lacquers applied by dipping or electropainting
- electropainting in conjunction with phosphating
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 299
Projects
Change of existing galvanization plants to meterials loss minimized process technique at simultaneous costs reduction: Change of a hot-dip galvanising (TV 16)
- optimization of the degreaser and development of a regenerator for the discharge of oil/grease from the degreaser solution for unlimited bath life: cost reduction of about 6,000 EUR/year
- installation of a cross-flow/counter flow heat recovery plant and further energy saving measures: energy savings of about 150-170 kWh (= 30-35%)
- measures for savings in materials through "Partial Automation Galvanizing Kettle", "Application of New Zinc Alloys", "Installation of a Zinc Ash Treatment Plant", "Application of a Vibration Hard Zinc Grab", and other peripheral individual measures: savings in zinc compared to the initial situation of about 17% as well as additionally about 12,000 €/year due to other effects
- the application of the ion exchange process enables the zinc-elimination from the mixed acid pickles; with this, logistical advantages (higher plant productivity) are utilized and internal cycles are being closed: savings potential of about 50,000 EUR/year
Development of a process for cyanide-free alkaline bright zinc plating in conventional galvanic plants and the substitution of chromium (VI) in zinc passivation
- processes in centrifugal systems show better corrosion protection compared to conventional cyanide-free processes
- compared to conventional systems with comparable capacity, carry-over in centrifugal systems is only at about 10-25%, since the solution is centrifuged and recirculated after the treatment
Pickling and roasting of semi-finished components of copper and cupro-nickel composition without the use of nitric acid and chromic acid
- replacement of chromium acid and nitric acid as pickling solutions
- continuous regeneration of the pickling solution
- copper recovery by means of electrolysis resp. membrane electrolysis
- waste water-free process
Reorganization of galvanic plants to material loss minimised process techniques with simultaneous reduction of costs: Increase in efficiency by inclusion of a central chemical-physical treatment plant
- production-integrated processes for the reduced consumption of raw materials and waste avoidance considerably reduce the expenses for ensuing cleaning and disposal measures
- the connection of a mobile ion exchanger system with electrolysis cells for the field of electroplating provides cost-effective waste water treatment and high-quality recycling of wastes
Substitution of galvanic processes using secondary plasma flame coating
- Ion source with deflectable beam for secondary ion beam coating as an alternative to electroplating
- no toxic waste water or acid or base vapour in ion beam coating due to the elimination of chemical pre-cleaning steps and subsequent treatment
- the coating rates of chromium are higher than those of electroplating
- the ion beam coating method influences the structure of the deposited layers to a far greater extent and prevents columnar crystal formation
Feeding / Handling
Best Available Techniques
Appropriate treatment of workpieces or substrates
Correct treatment of workpiece or substrate surfaces with appropriate specification can avoid stripping and rectification of significant amounts of metal.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 199, p. 200
Optimized barrel processing reduces drag-out
The plastic material of the barrel normally has a smooth surface and is inspected for worn areas and the formation of recesses or bulges around the holes. The bores of holes in the panels usually have a sufficient cross-section to minimise capillary effects, and the thickness of the panels of the cylinder is just thick enough to meet the mechanical strength requirements. The total proportion of the body of the barrel that is perforated is usually as high as possible to allow the drag-out to drop back easily into the process tank. This also improves the efficiency of the whole plating process by allowing easier solution access and decreasing voltage drop. A further reduction of drag-out can be attained by intermittent rotation of the barrel above the process tank while draining (such as rotating for about 90 degrees, stopping for at least 10 seconds, next sequence of intermittent rotation, etc.). More reduction of drag-out can been achieved by the application of draining ledges within the barrels to allow the draining liquid to flow together and to drain out of the rotating barrel. Drag-out can be reduced dramatically by blowing excess solution out of the barrel while draining over the bath. With hot baths, the barrels can be rinsed with water or sprayed, although for barrels, sparging is more effective: sparging is where pipe is constructed within the barrel and runs rinse-water inside the barrels and through the workpieces. In a barrel, the workpieces usually lie with the main surfaces horizontal. To achieve better draining, inclined lifting of the barrels from the tanks can be considered. The suspension and hoisting systems may be adapted to this requirement. However, in conventional systems this is difficult to achieve.
The application of mesh plugs instead of holes has proven successful, by reducing the length of
the bores in the panels of the cylinder body of the barrel. The drag-out can be decreased, and the
voltage drop at the perforation is effectively reduced.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 229
Optimized jig (rack) processing reduces drag-out
Arrange the largest surfaces of the workpieces in a vertical position at the jigs (racks) allows the adhering solution to run down to the bottom edge of the workpieces.When lifted out of the process solution, the jigs may be tilted in such a way that large droplets can be formed faster and drip down from the lowest point of the suspended articles. Allow sufficient drainage time above the process tank to let the adhering liquid to cohere and form droplets which will drip from the articles. Allow sufficient drainage time above the process tank to let the adhering liquid to cohere and form droplets which will drip from the articles. By slow withdrawal of the jigs from the process solution, the drag-out volume can be decreased considerably. Cup-shaped recesses are normally avoided where possible, and cup-shaped components are jigged cup-side down on the incline so process solution is not carried into the rinse-water. In some cases, arrangements can be made by dialogue with customers for components with high drag-out retention, such as cup-shaped components, to be manufactured with drainage holes. Dripping of process solution on other articles arranged lower on the jig is normally addressed by suitable positioning of the workpieces. Drag-out by jigs can be reduced by inclining supporting arms to avoid horizontal surfaces from which the adherent solution cannot easily run off. A normal inspection and maintenance task is to check the insulation coating of the jigs to ensure smooth surfaces, with no fissures or cracks in damaged insulation to trap and retain solution. It is good practice to regularly inspect jigs for defective insulation so they can be identified for replacement or repair
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 229
Rinsing of jigs (racks) or barrels reduces drag-out
When jigs (racks) or barrels are being removed from a tank of heated solution, it is good practice to drench it with a fog spray while it is still over the processing tank. This achieves a reduction in drag-out loss, and the water used compensates for evaporation. This treatment can be combined with a pre-rinse, returning water from the first static rinse to the process solution. For removing solution adhering to, or trapped in, recesses, combined water and air jets may be used above the process tank and within an empty tank, respectively.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 229
Projects
Preventing waste by using a new pretreatment method in galvanizing plants for the galvanization of parts
- lifting frames for all process baths of the pretreatment line: reduction of the hydrochloric acid concentration by means of intermittent lifting movements of the goods and thus lower carry-over rate in subsequent baths
- low hydrogen chloride emissions at the workplace
- the dust accruing when cleaning the galvanizing bath from exhaust gas by means of a separator can be easily processed for the recovery of flux salts
- dustlike emissions in the cleaned exhaust gas only reach 1/25 of the permissible upper limit of 10 mg/cbm; in case of cadmium, they are below the detection limit of 0.0002 mg/cbm
Rinsing
Best Available Techniques
Chemical rinses
- An accelerated technique to achieve the required cleaning efficiency is obtained by means of chemical rinses called the Lancy process. Here the dragged-out process solution is reacted chemically with the rinsing liquid at the same time.
- Reduces the effluent treatment capacity required by reducing or eliminating the primary waste water treatment stages.
- Incorporation of chemical rinsing can reduce the number of rinse stations with a consequent increase in contaminated rinsing.
- The main use of the Lancy process, the oxidation of dragged-out cyanides by rinsing in chlorine bleaching caustic solution, is now reduced because of concerns about the associated AOX generation.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 239, p. 242
Drag-in reduction
Drag-in can contaminate a process solution if is there is insufficient rinsing after the previous processes. Drag-in of clean rinse-water can significantly dilute a process solution. Drag in can be minimised by using an eco-rinse (or pre-dip), see Section 4.7.4, or by removing as much rinse-water as possible, such as by air knives or wiper rollers for sheet or coil substrates. The effects can also be minimised by using compatible chemical systems Drag-in reduction leads to extending process solution life, improving process quality and reducing material costs in make up chemicals.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 229
Drag-out reduction
Measures for drag-out reduction:
- Transition from drag-out draining to rinsing: When jigs (racks) or barrels are being removed from a tank of heated solution, it is good practice to drench it with a fog spray while it is still over the processing tank. This achieves a reduction in drag-out loss, and the water used compensates for evaporation. This treatment can be combined with a pre-rinse, returning water from the first static rinse to the process solution. For removing solution adhering to, or trapped in, recesses, combined water and air jets may be used above the process tank and within an empty tank, respectively.
- The use of compatible chemicals (e.g. the use of the same acid in pickling or activating the surface prior to an acid-based plating process) reduces the consequences of chemical drag-over to the subsequent process.
- Arrange the largest surfaces of the workpieces in a vertical position at the jigs (racks) allows the adhering solution to run down to the bottom edge of the workpieces
- optimized barrel processing to allow the drag-out to drop back easily into the process tank. The barrels can be rinsed with water or sprayed, although for barrels, sparging is more effective.
- Properties of process solutions (e.g. Increasing the temperature of process solutions)
A reduction of drag-out is an effective primary measure for:
- minimising losses of chemicals in rinses
- reducing the amount of rinsing required
- reducing raw material costs
- reducing quality and maintenance problems with subsequent processes
- reducing environmental problems associated with rinsing waters.
Drag-out depends on a large variety of parameters and a reduction of this key step with many impacts on the environment and the process can only be achieved by close co-operation of all personnel involved. For this reason a thorough understanding of the complex interrelations of many parameters is needed by the operational staff to improve the situation successfully.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 229
Eco rinse or pre-dipping
Some drag-out from process solutions working at (but not limited to) ambient temperature can be recovered through a single rinse station in which the workload is dipped before and after being processed. The eco rinse station (or pre-dip) can be made up with diluted process solution from the very beginning or filled with deionised water only. In this case it will take some time until the final equilibrium concentration of 0.5 C0 (50 %) will be reached. The solution only has to be changed when the tank itself and/or the tank walls have to be cleaned. During normal operation, no water has to be added assuming that drag-in is equivalent to dragout. Drag-out recovery rate (jig and barrel plating) is approximately 50 %.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 239
Multiple rinse techniques
cascade rinse systems for a commensurate reduction in waste water volumes and treatment. Multiple stage rinsing is particularly suitable to achieve a high rinsing rate with a small amount of rinsing water. In cascade rinsing, the water flows in the opposite direction to the workpieces.a smaller rinsing quantity of water can be achieved by the selection of the correct rinsing system. The effect of water saving decreases with an increasing number of rinsing stages. However, the volume of water required decreases to the point where direct make up for water losses from process solutions at ambient temperatures can be considered. The achievable recovery rate is, at a given volume of evaporation, directly related to the concentration of process chemicals in the first rinse station. Closing the loop for a process requires the water returned to the process solution from the first rinse station to be brought into balance with the water lost in evaporation and drag-out. Process solutions operated at higher temperatures and with multi-stage rinsing offer possibilities for this. By the introduction of multistage rinsing systems partly combined with a rinsing water recycling system and other techniques and decreases of waste water of up to 90 % can be obtained. One coil coating plant reports a reduction of 30 m3 per hour.
- Multiple stage counterflow rinse
- Multiple static rinse
- Dual static rinse followed by final flow rinse with recirculated water
- Multi-cascade rinsing with limited process line space (cascades are external to the process linen due to lack of space; in the treatment line there is only one rinsing tank per process step. Each rinse tank is connected to several external tanks which work as rinsing stages according to the cascade principle. The workpieces or substrates are brought into the rinsing tank and rinsed successively with the water from the individual rinsing stage tanks, becoming progressively cleaner. Rinsing can be by sprays or filling the tank to immerse the workpieces or substrates.)
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 245, p. 252, p. 340
Regeneration and re-use/recycling of rinsing water
Spent rinse-water can be regenerated and this can lead to savings in water consumption and will reduce to amount of waste water to be treated, reducing the waste water treatment costs for capital investment, energy usage and chemicals.
- By feeding the rinsing water through cation and/or anion exchangers, the cations become exchanged for H+, and the anions for OH, and water of a quality approaching demineralised water is achieved. This is fed back to the rinsing system.
- Regeneration by reverse osmosis: used to concentrate rinse-waters and recover materials, treat waste waters and incoming or recycled water. Reverse osmosis uses a hydrostatic pressure gradient across a semi-permeable membrane to separate water from a solution of salts. The pressure applied exceeds the osmotic pressure of the feed solution causing water to flow from the concentrated solution to the more dilute solution: the reverse of the natural osmotic diffusion. Dissolved solids are rejected by the membrane surface. Many multi-charged ions can be rejected at rates exceeding 99 %. Singlecharged ions typically have rejection rates in the range of 90 - 96 %.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 243, p. 261
Rinsing of jigs (racks) or barrels reduces drag-out
When jigs (racks) or barrels are being removed from a tank of heated solution, it is good practice to drench it with a fog spray while it is still over the processing tank. This achieves a reduction in drag-out loss, and the water used compensates for evaporation. This treatment can be combined with a pre-rinse, returning water from the first static rinse to the process solution. For removing solution adhering to, or trapped in, recesses, combined water and air jets may be used above the process tank and within an empty tank, respectively.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 229
Rinsing stages using recycled water
The technique may be regarded as an extension of the integrated treatment system (widely known as the Lancy system). Water from a rinsing stage is re-used in another rinsing stage, where the chemical or physical characteristics acquired in the first stage can be exploited in the second stage without requiring any additional treatment.
- Reduction of water consumption up to 40 %
- Reduction of chemicals used to modify the pH of water after the rinsing stages
- Reduction of chemicals used to neutralise the water before to channel it into the treatment plant
The technique applies only to processes free of cyanides.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 228
Rinsing techniques for manual or semi-automatic lines
are used for small production throughput, or for developmentwork. Controlling water usage, drag-in and drag-out may appear more difficult. To achieveadequate draining time on a manual line, the jig or barrel should be supported on a static rackover the preceding bath. This enables spray rinsing to be carried out directly above the treatment tank to return the drag-out and/or allows draining to be properly timed before immersion in a rinse. In semi-automatic lines, spray rinsing can be also carried manually.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 239
Single rinsing techniques
In certain situations, single rinsing operations are necessary. This may be where there is a loss in quality if there is too much rinsing of the surface, for example, black passivation zinc, thick film passivations or rinsing between nickel and bright chromium. In other cases, the stopping of the surface reaction succeeds only when there is quick dilution in the first rinsing stage, which requires the use of high quantities of water. In such cases, the concentration of the reacting chemicals in the first rinsing stage must kept low. Other examples are manual or semi-automatic lines with small production throughput, or used for development work.
To minimise environmental impacts:
- water used here may be regenerated and recycled within the process, e.g. by a deioniser, or water regenerated from elsewhere may be used
- where technically possible, having compatible chemistry for the preceding and subsequent solutions can minimise the need for rinsing (e.g. same acid base)
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 244
Spray rinsing
Spraying prior to rinsing (or pre-rinse) above the process bath is an effective method of rinsing. The rinsing water is sprayed onto the workpieces while they are still above the bath surface. This may be manually for small process lines, or automatically. For the pre-rinse, the amount of water to be used should equal that dragged-out from the process tank to maintain the water balance. The pre-rinse causes a direct feedback of process solution into the process tank. Spray rinsing in a separate tank acts as a first rinse. The solution can then be recycled back to the process solution in amounts equal to evaporative and drag-out losses.
This is a key step in reducing the loss of soluble chemicals from process vats to the environment
via rinsing.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 239
Projects
Completion of the material cycle in procedures for the removal of materials in process solutions: membrane application technology and testing in a hot-dip galvanising plant
- electrodialysis for rinsing water treatment
- energy requirement for the treatment of 1.4 m³/h is 0.5 kW/h
Reorganization of galvanic plants to material loss minimised process techniques with simultaneous reduction of costs: Liebherr-Aerospace Lindenberg GmbH
- gradual conversion of core plant and peripheral facilities into process technology with minimized material losses during production
- optimized rinsing technology
- realization of material loss minimization, cost reduction and reduction of waste water volume
Reorganization of galvanic plants to material loss minimised process techniques with simultaneous reduction of costs: rack for automatic metal surface finishing
- increased recirculation rate and significant reduction of the consumption of process chemicals and chemicals for waste water treatment through optimized rinsing technology in connection with an ion exchanger recirculation plant
- closed internal material cycle without peripheral concentrator and regenerator for the subsystem of chromium
Sewage and waste reduced electroplating plant through water recycling and producing of secondary raw materials
- when degreasing, additional pre-degreasing is carried out exclusively with hot water in order to save detergents
- clocked spray rinsing and recirculation enabled the reduction of waste water to 20-30% in comparison to conventional plants
- regeneration of cleaning baths by means of micro-filtration and reverse osmosis
- deoxidation baths are being concentrated through reverse osmosis and added to the pickling bath
- exhausted pickles are being processed through the separation of the contained metals (iron and zinc) by means of ion exchangers
- non-recoverable metals (iron, chromium and zinc of chromating baths) are being selectively separated through gradual precipitation at different pH-values and are then processed
- waste waters from phosphating are separately collected and treated; residues are fed to zinc slurry treatment
- process solutions from demetallization are separately electrolytically processed
- the zinc and nickel containing solutions of the electrolytical metal deposition are recirculated in an almost closed cycle
- energy-saving concentration of the rinsing waters to be recycled is carried out by means of reverse osmosis and evaporation
Substitution of galvanic processes using secondary plasma flame coating
- Ion source with deflectable beam for secondary ion beam coating as an alternative to electroplating
- no toxic waste water or acid or base vapour in ion beam coating due to the elimination of chemical pre-cleaning steps and subsequent treatment
- the coating rates of chromium are higher than those of electroplating
- the ion beam coating method influences the structure of the deposited layers to a far greater extent and prevents columnar crystal formation
Sourcing and storage of material
Best Available Techniques
Corrosion prevention coating with oil or grease
- Oil and/or grease may be used for corrosion prevention during storage. The disadvantage is that items have to be cleaned.
- Prevention of reworking and scrapping
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 214
Packaging
Workpieces or substrates may be packed with absorbent or corrosion preventing materials such as specialist papers or woodchips.
- prevent corrosion and surface damage in transit
- increased consumption of raw materials can be offset by selecting and using recyclable packaging systems
- reduced stripping and reworking.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 213
Shortening storage time
Eliminating or shortening storage between operations, e.g. between manufacture and surface treatment, or between surface treatment and dispatch, can avoid the need for other corrosion prevention treatment.
- realisation via JIT (just in time) system or good production planning
- prevention of stripping and reworking
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 212
Storage of chemicals and workpieces/substrates
- avoid generating free cyanide gas by storing acids and cyanides separately
- store acids and alkalis separately
- reduce the risk of fires by storing flammable chemicals and oxidising agents separately
- reduce the risk of fire by storing any chemicals which are spontaneously combustible when damp, in dry conditions and separately to oxidising agents. Mark the storage area of these chemicals to avoid the use of water in fire-fighting
- avoid the contamination of soil and water environments from spillages and leakages of chemicals
- avoid or prevent the corrosion of storage vessels, pipework, delivery systems and control systems by corrosive chemicals and fumes from their handling prevent degradation of metal workpieces/substrates in storage by one or a combination of:
- shortening storage time
- controlling the corrosivity of the storage atmosphere by controlling the humidity, temperature and composition
- using either a corrosion preventing coating or corrosion preventing packaging.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 394
Projects
Conversion to material loss minimised process technology under simultaneous cost reduction: examplewise compilation of new forms for the cooperation between plating shop and supplier
web-enabled, graphically structured manual with specific decision guidance for the conversion of existing electroplating systems to process technologies with the lowest possible consumption of chemicals for the cooperation of plating shops and suppliers of chemicals
To complete Project DescriptionReorganization of galvanic plants to material loss minimised process techniques with simultaneous reduction of costs: involvement of a specialised firm from the process chemical sector
- minimization of material losses through new forms of cooperation and billing of services between operators of electroplating plants and companies specialized in process chemistry
- the calculation based on process indicators gives the specialized company a share in the success of the saving measures through the definition and monetary assessment of success criteria
Subsequent treatment / rework
Best Available Techniques
Process Specification
- Application of adequate specification to avoid reworking
- cost savings in raw materials, hazardous waste disposal, energy and water, as well as labour
- Avoiding reworking minimises losses in raw material, energy and water inputs, as well as minimising waste water treatment and the generation of sludge and liquid acid wastes.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 212
Quality Management Systems QMS
- Reduction in reworking and scrap can be achieved by using formal quality mamangement systems (QMS)
- Avoiding reworking minimises losses in raw material, energy and water inputs, as well as minimising waste water treatment and the generation of sludge and liquid acid wastes.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission Chapter 4.1.1.+ p. 200
Reduction of Stripping
Workpieces or substrate surface treated incorrectly, to the wrong or inappropriate specification, or a specification incorrectly applied can lead to significant amounts of metal stripping and rectification.
Reduction in this activity can result in reductions in:
- spillage which can attack concrete floors and can pollute surface and groundwaters
- exceeding waste water treatment plant capacity leading to breach of permit discharge conditions
- acid fumes and mists leading to problems with local air quality, health and safety and deterioration of plant and equipment.
Reduction in surface treated scrap can reduce emissions to air from other installations such as furnaces and foundries. In these, some non-ferrous metals may be vaporised and organic coatings burnt off with unknown breakdown products.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 199, p. 200
Exhaust air treatment
Projects
Preventing waste by using a new pretreatment method in galvanizing plants for the galvanization of parts
- the dust accruing when cleaning the galvanizing bath from exhaust gas by means of a separator can be easily processed for the recovery of flux salts
- dustlike emissions in the cleaned exhaust gas only reach 1/25 of the permissible upper limit of 10 mg/cbm; in case of cadmium, they are below the detection limit of 0.0002 mg/cbm
- lifting frames for all process baths of the pretreatment line: reduction of the hydrochloric acid concentration by means of intermittent lifting movements of the goods and thus lower carry-over rate in subsequent baths
- low hydrogen chloride emissions at the workplace
Waste and waste water treatment
Best Available Techniques
Anodising caustic etch recovery
Regeneration can reduce a plant’s solid waste by over 80 % while lowering caustic chemical (and neutralisation) costs by over 70 %. The removed alumina crystals may be used in a variety of alumina substitutes. A hot solution of sodium hydroxide creates a decorative matt surface finish by removing a thin layer of aluminium. The etching process is typically responsible for 80 - 90 % of the aluminium in the waste treatment system. Chemical stabilisers (complexing agents) are added to prevent the aluminium from precipitating out in the etch tank. Water is used to rinse the etching solution off the parts. The rinse-water carries dissolved aluminium and caustic to the plant waste treatment system. If stabilisers are not used, the sodium aluminate concentration becomes too high and it will hydrolyse to produce alumina trihydrate, liberating free caustic soda. This reaction is used in the primary aluminium industry to make alumina. If not properly controlled, it leads to an accumulation of a rock-hard aluminium hydroxide scale in the etch tank. A regeneration system recirculates the etch solution continuously between the etch tank and a separate crystalliser tank, where the etch solution is seeded with alumina crystals in a separate crystalliser tank. It is then possible to regenerate the etch solution without scale building up. The hydrated alumina crystals formed in the crystalliser settle out in a settlement section. Regenerated etch solution, with reduced aluminium and increased free caustic levels, feeds back to the etch bath directly from the top of the crystalliser. Alumina crystals are withdrawn periodically from the bottom of the crystalliser and dewatered in a vacuum filter.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 313
Chemical rinses
- An accelerated technique to achieve the required cleaning efficiency is obtained by means of chemical rinses called the Lancy process. Here the dragged-out process solution is reacted chemically with the rinsing liquid at the same time.
- Reduces the effluent treatment capacity required by reducing or eliminating the primary waste water treatment stages.
- Incorporation of chemical rinsing can reduce the number of rinse stations with a consequent increase in contaminated rinsing.
- The main use of the Lancy process, the oxidation of dragged-out cyanides by rinsing in chlorine bleaching caustic solution, is now reduced because of concerns about the associated AOX generation.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 239, p. 242
External re-use and recycling of waste
Wastes that cannot be recovered internally may be valorised externally by third parties. To assist with this, it may be good practice to keep these waste streams separate to maintain a concentration of components that makes recovery viable or to prevent contamination, such as contamination of aluminium hydroxide sludge with heavy metals.
Examples of external valorisation:
- hydro and pyrometallurgical companies engaged in non-ferrous metal refining. Part of the electroplating sludge may have a high value material content and recycling by third parties can be arranged in many cases. Recycling includes refining of the metals copper, nickel, chromium and zinc from suitable electroplating sludge as metals or metal compounds
- manufacture of usable metal concentrates
- phosphoric and chromic acids, spent etching solutions, etc.
- aluminium hydroxide from anodising can be precipitated and recycled, for example as a coagulant for sewage treatment. (Note: the rinsing waters from colouring and sealing processes may contain heavy metals and it is advisable to collect sludge separately from these waste water streams if re-use is required)
- inorganic chemical companies and the glass and ceramics industry which use metals or metal compounds intentionally in the manufacture of products.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 379
Regeneration and re-use/recycling of rinsing water
Spent rinse-water can be regenerated. This can lead to savings in water consumption and will reduce to amount of waste water to be treated, reducing the waste water treatment costs for capital investment, energy usage and chemicals.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 243, p. 261
Waste minimisation and avoidance
There are four key factors for the avoidance and minimisation of waste in surface treatment
processes:
- reducing the amount of hazardous material in the waste
- extension of the service lifetime of the process solution
- decrease of the drag-out of process solutions
- feedback of the dragged-out process solutions into the process tanks
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 379
Projects
Avoidance of Wastes by Low-waste Production Procedures - Electroplating
- establishment of waste concepts: adaptation of rinsing criteria to the actual demand and reduction of drag-outs through the optimization of the draining process
- avoidance of slurry production of the process steps of up to 40%
Changing existing galvanization plants to materials loss minimized technique at simultaneous reduction: Galvanotechnik Breitungen
- reduction of material losses in the metallization of plastics (layer series copper/nickel/chromium) through the change of process and plant technology, the optimization of volume flows and material flow control measures
- cost reduction through the development of internal material and water cycles
- material cycles with recirculation rates of about 90% through the use of evaporators and regenerators
- reduction of material consumption (chemicals, water) and the amount of waste water and waste
Closing circuit of contaminated process streams: fundamental development of process technologies
- nanofiltration for halogenide separation from strongly alkaline solutions
- electrodialysis for chloride separation from sulphuric solutions and for the treatment of halogenide-containing washing acids
Closure of material cycles by the application of degradation in process solutions: developments for the material recycling of residues containing copper and nickel
- optimization of smelting and recycling processes
- significant increase in the recycling rate of waste from galvanizing plants
Construction, testing and optimization of a low-waste electroplating plant operated without waste water to be drained
- waste water-free operation of the plant due to recycling of production chemicals and recirculation of auxiliary production materials
- the small residual amount of production waste water is vaporized in an energetically optimized evaporation plant; in this process, distilled water is extracted which is then returned to the process in the form of rinsing water
- reduction of residues: electroplating slurries by 75%, phosphating sludges by about 40%; acids, alkaline solutions, chromating solutions and cyanidic solutions were completely eliminated from the residue balance
- the operating costs, which are slightly higher than those of a conventional plant, are accompanied by considerable savings in disposal costs, procurement costs for raw materials (input of metal and chemicals) and energy costs.
Cyanide degradation and the biosorption of heavy metals in waste waters from ore mining and treatment works
- biotechnical plant for cyanide degradation by means of bacteria and heavy metal sorption in fungi
- avoidance of accumulations of toxic intermediate and final products at low operating costs
Development of an environmentally friendly technology for metal chemistry and electroplating
- minimization of the carry-over of process solutions in rinsing processes by means of new geometries and surfaces of transport baskets
- no modification of existing plant required
- regeneration method for a particular tin-nickel electrolyte enabling a nearly complete closure of the material cycle in this sub-process
- complete separation of recyclables and decomposition products (contaminants) through evaporation
Reorganization of galvanic plants to material loss minimised process techniques with simultaneous reduction of costs: Increase in efficiency by inclusion of a central chemical-physical treatment plant
- production-integrated processes for the reduced consumption of raw materials and waste avoidance considerably reduce the expenses for ensuing cleaning and disposal measures
- the connection of a mobile ion exchanger system with electrolysis cells for the field of electroplating provides cost-effective waste water treatment and high-quality recycling of wastes
Substitution of galvanic processes using secondary plasma flame coating
- Ion source with deflectable beam for secondary ion beam coating as an alternative to electroplating
- no toxic waste water or acid or base vapour in ion beam coating due to the elimination of chemical pre-cleaning steps and subsequent treatment
- the coating rates of chromium are higher than those of electroplating
- the ion beam coating method influences the structure of the deposited layers to a far greater extent and prevents columnar crystal formation
Process peripherals and superordinate measures
Best Available Techniques
Benchmarking the installation
- establish benchmarks (or reference values) that enable the installation’s performance to be monitored on an ongoing basis and also against external benchmarks
- continuously optimise the use of inputs (raw materials and utilities) against benchmarks. A system to action the data will include:
- identifying a person or persons responsible for evaluating and taking action on the data
- action being taken to inform those responsible for plant performance, including alerting operators, rapidly and effectively, to variations from normal performance
- other investigations to ascertain why performance has varied or is out of line with external benchmarks.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 393
Benchmarking water consumption
Reducing water flow through the process is crucial, not only for water saving, but can be used with drag-out controls to reduce raw material usage and increase materials efficiency. This also reduces the size of waste water treatment plant needed and the treatment chemicals and energy used in treatment.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 205
Environmental management
Implementation of an Environmental Management System (EMS) that
incorporates the following features:
- definition of an environmental policy for the installation by senior management
- planning and establishing the necessary procedures
- implementation of the procedures, paying particular attention to structure and responsibility, training, awareness and competence,communication,employee involvement, documentation, efficient process controls, maintenance programmes, emergency preparedness and response, safeguarding compliance with environmental legislation
- checking performance and taking corrective action, paying particular attention to monitoring and measurement, corrective and preventive action, maintenance of records, independent internal auditing in order to determine whether or not the environmental management system conforms to planned arrangements and has been properly implemented and maintained
- review by senior management
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 392
Shortening storage time
Eliminating or shortening storage between operations, e.g. between manufacture and surface treatment, or between surface treatment and dispatch, can avoid the need for other corrosion prevention treatment.
- realisation via JIT (just in time) system or good production planning
- prevention of stripping and reworking
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 212
Storage and transport conditions
store components - both awaiting treatment and treated - away from humid and acid air:
- good ventilation of the workplace will assist, as will ensuring the vented moist, and often acid, exhaust air does not come into contact with products in storage or awaiting transport
- ventilation of the storage areas
- keeping products warm in transport and storage to avoid moisture condenses on components
- reduction of stripping and reworking
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 212
Substitution – choice of raw materials and processes
Reasons for using less harmful substances:
- lower materials usage, both in process, in waste water and air emission treatment
- energy saving
- water saving
- improved quality and reliability of the process
- costs savings for decreased waste water treatment
- cost savings for reducing air extraction an treatment
- health and safety in the workplace.
Substitution can be achieved by:
- substitution directly of one substance by a less harmful one. An example is the replacement of EDTA or NTA with derivatives of gluconic acid. There are limited opportunities for this in surface treatment
- substitution by different process chemistries or methods. This is used where there is no direct replacement, for example, replacement of zinc cyanide by cyanide-free alkali or zinc acid solutions. Different coating process chemistries give treatments with different properties, even for the same materials
- substitution by different surface treatments, such as substituting autocatalytic nickel or vapour deposition of chromium for hard chromium plating. If the substitution is for the core treatment, the final properties may be different.
source:
- European Commission (2006): Integrated Pollution Prevention and Control, Reference Document on Best Available Techniques for the Surface Treatment of Metals and Plastics [online]. European Commission p. 271
Projects
Change of existing galvanization plants to meterials loss minimized process technique at simultaneous costs reduction: Change of a hot-dip galvanising (TV 16)
- optimization of the degreaser and development of a regenerator for the discharge of oil/grease from the degreaser solution for unlimited bath life: cost reduction of about 6,000 EUR/year
- installation of a cross-flow/counter flow heat recovery plant and further energy saving measures: energy savings of about 150-170 kWh (= 30-35%)
- measures for savings in materials through "Partial Automation Galvanizing Kettle", "Application of New Zinc Alloys", "Installation of a Zinc Ash Treatment Plant", "Application of a Vibration Hard Zinc Grab", and other peripheral individual measures: savings in zinc compared to the initial situation of about 17% as well as additionally about 12,000 €/year due to other effects
- the application of the ion exchange process enables the zinc-elimination from the mixed acid pickles; with this, logistical advantages (higher plant productivity) are utilized and internal cycles are being closed: savings potential of about 50,000 EUR/year
Reorganization of galvanic plants to material loss minimised process techniques with simultaneous reduction of costs: economic feasibility studies
production-integrated optimization approach for material-loss minimized process technology for existing (old) plants
To complete Project DescriptionReorganization of galvanic plants to material loss minimised process techniques with simultaneous reduction of costs: employing of an unbaised equipment contractor
- reduced expenses for ensuing cleaning and disposal measures through the application of production-integrated processes for the reduction of raw material consumption and the avoidance of wastes
- separation of the conceptual design of the technical process concept from the development and design of the plants
Reorganization of galvanic plants to material loss minimised process techniques with simultaneous reduction of costs: environmental and economic assessment and optimisation: ecological and economic evaluation and optimisation
life-cycle assessments as well as cost accounting and capital budgeting for the evaluation of optimization concepts and realized plant conversions
To complete Project DescriptionReorganization of galvanic plants to material loss minimised process techniques with simultaneous reduction of costs: solutions of automation at conversion of process engineering
- manual "Integrated Process Automation" for process engineers
- simulation model library for accelerated modelling and simulation of fundamental processes





